Organic electrodes for rechargeable batteries
There is great interest in developing organic electrodes for rechargeable Lithium and Sodium ion batteries. One of the unique features about organic electrodes is their design flexibility at the molecular level and good resource renewability. Organic compounds can be tuned through the modification of the structures as well as the introduction of functional groups. There have been significant developments in carbonyl based organic materials for batteries. In collaboration with Prof. Mahesh Hariharan’s research group at IISER Thiruvananthapuram, we’ve been looking at polyimide based organic electrodes over the several years. There are several issues with these systems, for instance, poor stability of the electrode upon continued charge and discharge cycles, mostly arising from their solubiity in organic solvents and their poor electronic conductivities. We have developed techniques to overcome these issues through polymerization of the material. Another major concern with organic electrodes is their low reduction potentials, compared to their inorganic counterparts, which still remains a challenge. Some of our recent research activities in this area are listed below:
I. Perylene-Polyimide based organic electrode materials for rechargeable lithium batteries
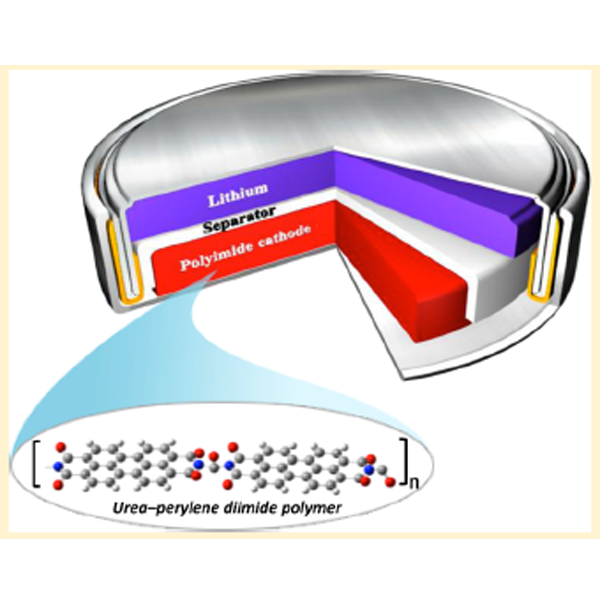
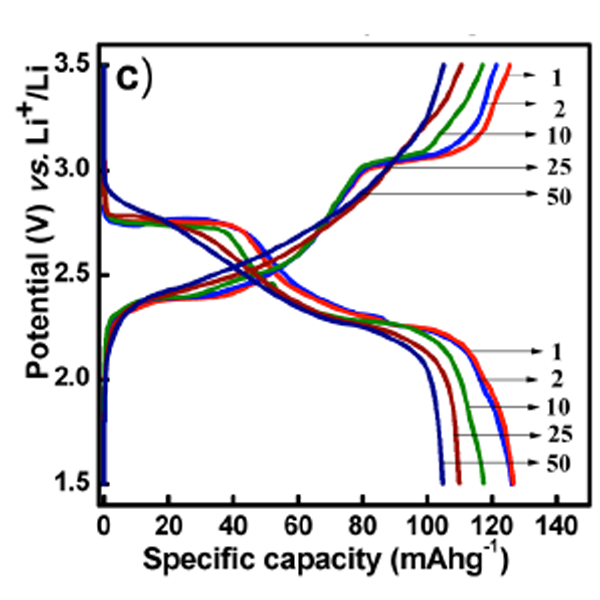
We studied perylenetetracarboxylicacid-dianhydride (PTCDA) polyimide-based organic electrodes for lithium-ion batteries. Detailed studies of the electrochemical behavior of polyimides of PTCDA synthesized using ethylenediamine, hydrazine, and urea as cathodes for rechargeable lithium battery applications are reported. We show that these novel polymeric carbonyl derivatives can be reversibly cycled at high current rates. Our approach to use the additional carbonyl group from urea polymerization resulted in improved lithium storage and better cyclic stability.
[Pavan Sharma, Dijo Damien et al., J. Phys. Chem. Lett 4, 3192-3197 (2013)] Read Article
II. Polyimide based all-organic sodium ion battery
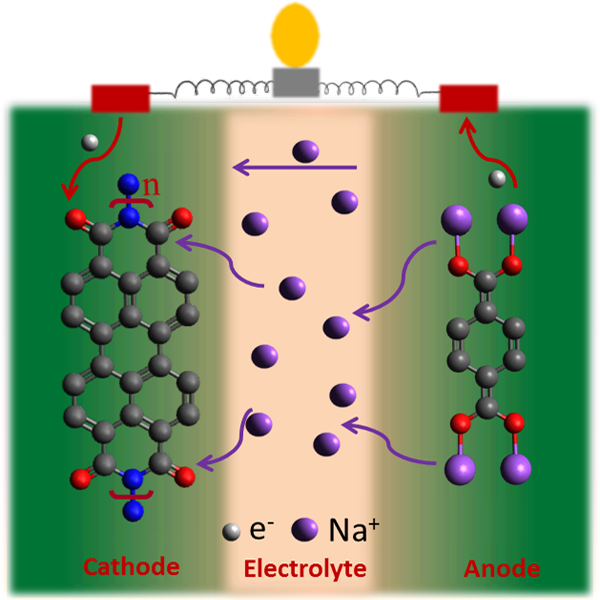
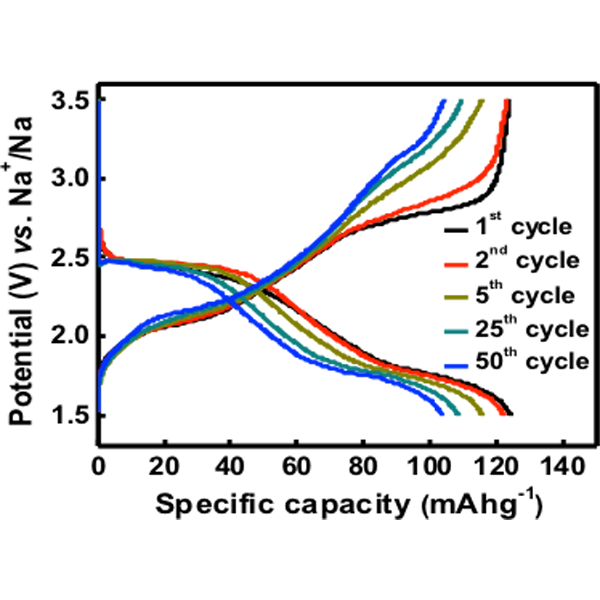
Developing new approaches to improve the performance of organic electrodes for rechargeable sodium batteries is important. In one of our recent studies, we explored N,N’-diamino-3,4,9,10-perylenetetracarboxylic polyimide (PI) as a novel cathode for a sodium battery and demonstrated an all-organic sodium ion battery using this polyimide as the cathode and disodium terephthalate (NaTP) (pre-sodiated) as the anode. The synthesised PI exhibits excellent electrochemical properties, when studied as the cathode for sodium batteries. The attractive electrochemical performance combined with the design flexibility of a PTCDA based PI material, offer new possibilities for the development of efficient all-organic sodium ion batteries.
[Harish Banda et al.,J. Mater. Chem. A 3, 10453-10458 (2015)] Read Article
III. Twisted Perylene Diimides with Tunable redox properties for Na-Ion Batteries
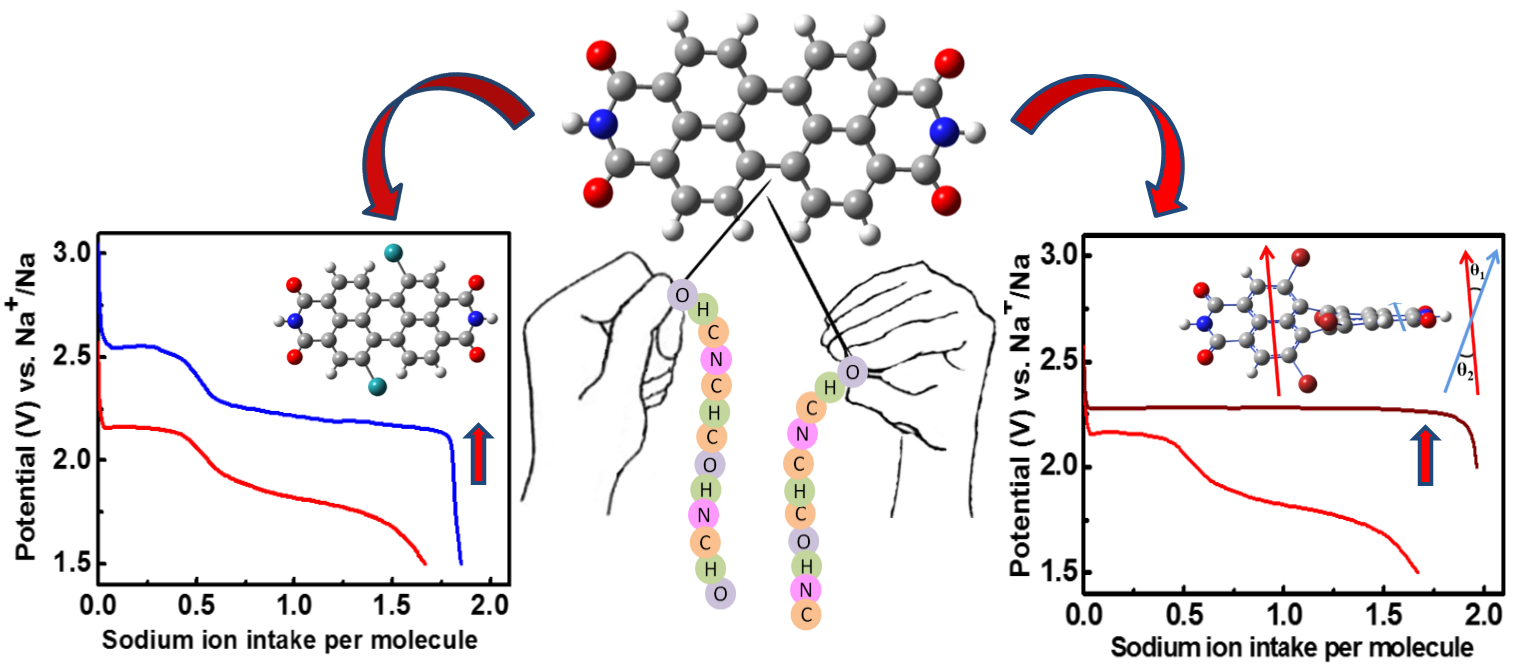
Organic rechargeable batteries gain huge scientific interest owing to the design flexibility and resource renewability of the active materials. However, the low reduction potentials still remain a challenge to compete with the inorganic cathodes. This study demonstrates a simple and efficient approach to tune the redox properties of perylene diimides (PDIs) as high voltage cathodes for organic-based sodium-ion batteries (SIBs). With appropriate electron-withdrawing groups as substituents on perylene diimides, this study shows a remarkable tunability in the discharge potential from 2.1 to 2.6 V versus Na+ /Na with a sodium intake of ≈ 1.6 ions per molecule. Further, this study explores tuning the shape of the voltage profiles by systematically tuning the dihedral angle in the perylene ring and demonstrates a single plateau discharge profile for tetrabromo-substituted perylene. Detailed structural analysis and electrochemical studies on substituted PDIs unveil the correlation between molecular structure and voltage profile. The results are promising and offer new avenues to tailor the redox properties of organic electrodes, a step closer toward the realization of greener and sustainable electrochemical storage devices.
[Harish Banda et al., Adv. Energy Mater. (2017), 1701316] Read Article